Introduction
Evolutionary speaking, the hypothalamus is a part of the so-called reptilian brain. Its core function is to integrate the most essential information about our homeostatic state from our periphery. Therefore, is the role of the hypothalamus tightly connected to our energy homeostasis, which is the most essential information for our survival.
Role Of The Hypothalamus in Food Behavior
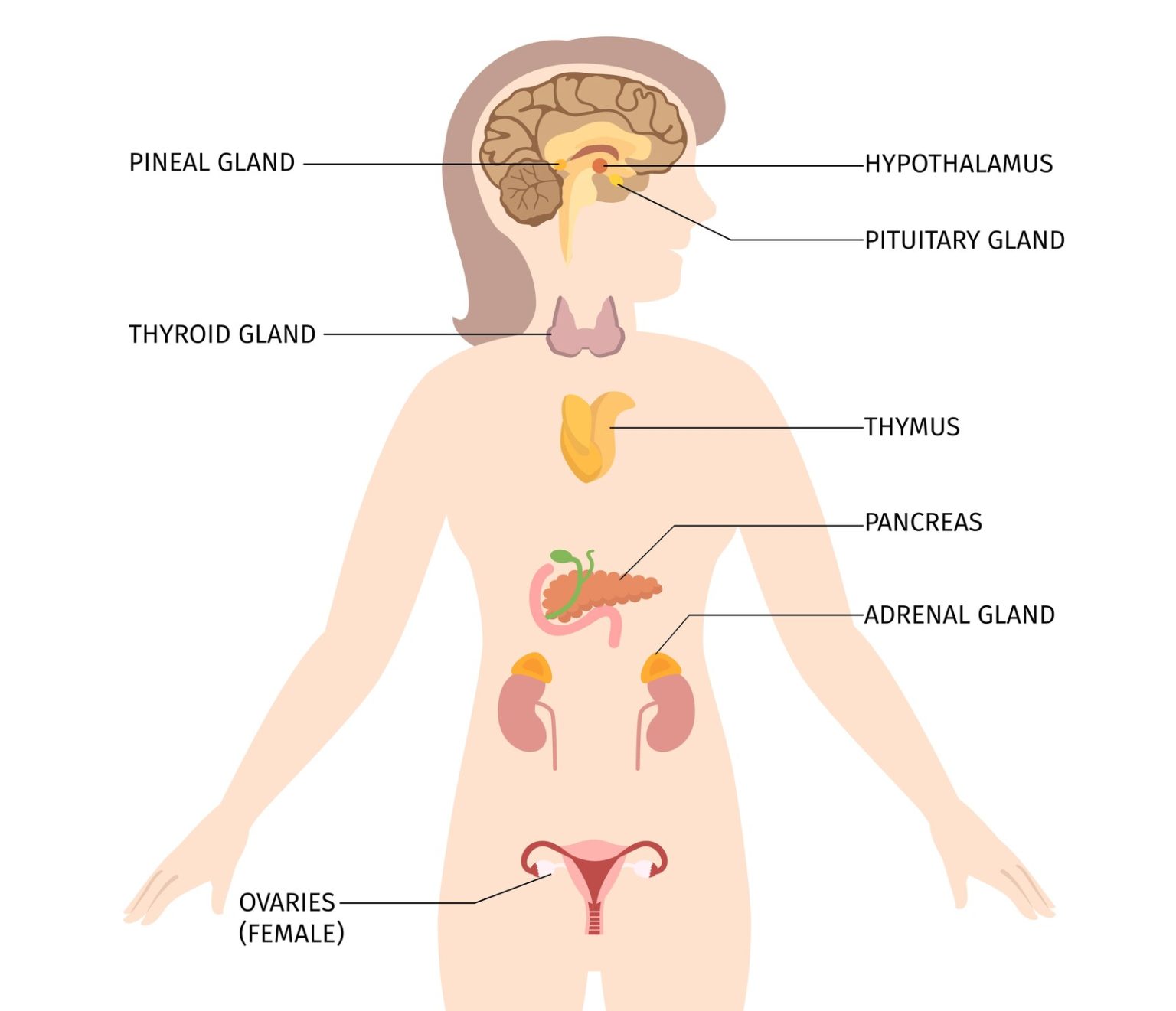
The hypothalamus represents the central brain unit responsible for the control over our endocrine system. The endocrine system is a collection of glands, which are responsible for the release of messenger molecules i.e. hormones. With that, the endocrine system allows our body to be aware of what is happening at different sites and what kind of response it is required to evoke – feedback loops.
By controlling the endocrine system, the hypothalamus modules hormonal release. The effects of the released hormones are of longer duration (hours and even days). When it comes to nutrition and food behavior, it is important to emphasize the following feedback loops or axes:
- Hypothalamus-pituitary-thyroid axis – HP TA and
- hypothalamus-pituitary-adrenal axis – HPA.
The HP TA axis is responsible for establishing energy equilibrium by modulating food behavior, while the HPA axis is responsible for modulating stress behavior [1]–[5].
Additionally, to the control of both axes, the hypothalamus plays a large role in modulating the reward system and higher-order cognitive structures. This connection between the more low-level reward systems with the higher-order cognitive structures implies that the hypothalamus serves as a gateway or relay component between survival mechanisms and motivational behavior.
hyperphagia - the first piece of the hunger puzzle
The involvement of the hypothalamus in food behavior has been established almost 80 years ago. Brobeck [6] found that lesions in the regions of the hypothalamus resulted in doubling the amount of ingested food (research was conducted on rats). In research, where food amount was controlled, it was noticed that the speed of ingestion was much faster, irrespective of the diet type. This research was one of the earliest, that defined the state of Hyperphagia – the state of extreme hunger. While enforcing eating behavior, lesions in the hypothalamus did not influence the efficiency of fatty acid metabolism. This led to the conclusion, that the hypothalamus itself is not involved in nutrient metabolism [6]–[8].
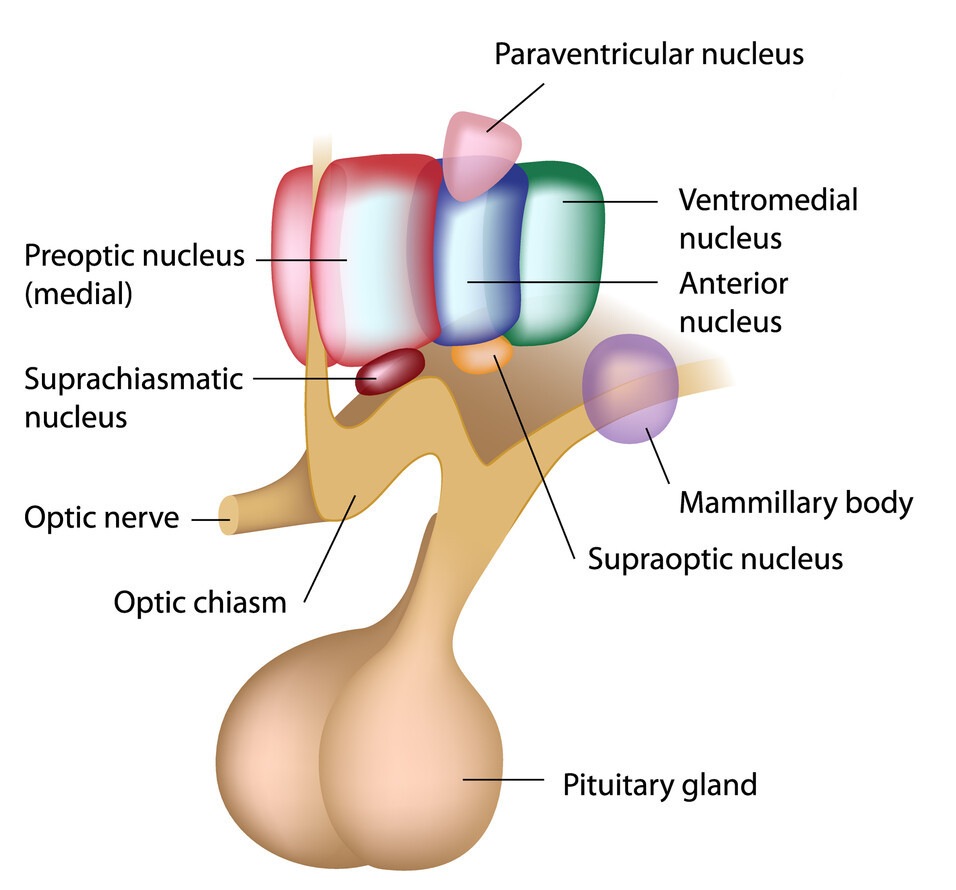
sensing the energy state of the organism
In later decades, the hunger modulating area has been more precisely located in the ventromedial lateral area of the hypothalamus [9]–[11]. Through that research, it was established, that the role of the hypothalamus is in relaying and integrating the homeostatic information to the motivation modulating areas in the brain.
Sensing of the homeostatic state is currently proposed through 3 theories:
- Glucostatic theory [12], [13]
- Lipostatic theory [14] and
- Setpoint theory [15].
All of these theories are complementary to each other and provide understanding as to how our brain, more specifically the hypothalamus, senses energy balance.
Conclusions
Through this blog post, we have established the role of the Hypothalamus as the essential hunger center, that controls all of our hormones and in that respect provides the strongest and most essential aspect of our drive for food behavior. However, in more (evolutionary) complex animals, food behavior is driven by other, higher-order structures which denote that the essential information about energy homeostasis is not the only one that influences our desires to eat or not to eat. Still, because of its essential and lower order function, the signals of the hypothalamus are the strongest and influence food behavior the most.