Introduction
Carbohydrates present the macronutrient with the strongest ability to promote the creation of fat reserves – lipogenesis. Before being stored in our body, carbohydrates need to be converted into their simple form – sugars. In that form, they can be used immediately as blood sugar i.e. glucose, combined into glycogen, or converted to other molecules like the triglycerides and lactate, which later continue to go through further metabolic processing.
The most prevalent sugars in our blood are glucose and fructose. They are metabolized through different pathways that produce different hormonal responses and metabolites. This effectively leads to a different fat accumulation pattern at different sites of our body.
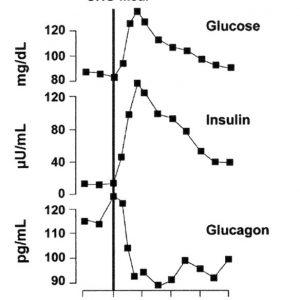
In the form of blood sugar, carbohydrates promote lipogenesis at different sites via different mechanisms, but predominantly through the hormone insulin. And the spike of insulin can be triggered even before the ingested carbohydrate is digested and absorbed into the bloodstream [1].
While there are differences in the rate of blood sugar influx amongst carbohydrate sources (measured with glycemic index and glycemic load), all of the broken down carbohydrates will eventually end up in the blood in their sugar form. In general, this will lead to elevated blood sugar and insulin levels and the production of cell-damaging species [2]. For that reason, a long term overexposure to higher intakes of carbohydrates can lead to peripheral as well as central tissue disorders [3]–[6].
Glucose and Increased Body Fat Storage
Glucose is the most abundant sugar. In animals, it is stored in a form of glycogen either in the liver to maintain stable blood sugar or in muscles to power semi intense muscular contractions. In our diet, we derive glucose mostly from plant-based sources, specifically in the form of starch from complex carbohydrates such as grains and tubers, as well as in the form of sucrose and glucose itself through the intake of fruits and vegetables.
Glucose storage: the tale of lacking space
In our body, glucose is used as an energy resource by various tissues. It is metabolized (broken down and used on-site) throughout the whole body, but mainly in the muscles and liver for glycogen storage, as well as the brain, and other organs. In the whole blood plasma of an average human body, there is storage capacity for 5-6 grams of glucose. In the form of glycogen, there is an additional 70 grams of storage in the liver and around 240 grams in the skeletal muscles. Because of the small storage capacity in the blood plasma, a very low amount of glucose is required to promote glucose partitioning i.e. storing it away for later use in the form of glycogen or converting them to other molecules such as lactate and triglycerides. This is a sharp contrast to gluconeogenesis, a process where the body synthesizes new glucose molecules from other substances. In that case, the rate of glucose production is demand-driven and so provides just enough of a glucose spike for the required task [7].
the glucose switch: locking away stored body fat
Working as a switch, the rise in blood glucose actively shuts down the process of fat metabolism i.e. lipolysis, while stimulating anabolic i.e. growth-stimulating processes like lipogenesis and glycogen synthesis. This means that fat burning is diminished or downregulated after a certain level of blood glucose has been reached (this however does not hold true for type 1 diabetics). When fat metabolism is slown down, clearance of lipids in the bloodstream and burning body fat reserves are diminished. This hormonal feedback loop is primarily guided through insulin, our primary anabolic hormone, which is secreted as a response to elevated blood glucose levels. Insulin is responsible for many vital functions such as glycogen synthesis (refueling glycogen reserves in our muscles and liver), lipogenesis (increasing fat reserves in our adipose tissues), and protein synthesis (buildup of new proteins) amongst others [8]–[11]. When our glycogen stores are filled up (total capacity around 300 grams for the whole body), the corresponding insulin response will predominantly promote lipogenesis at the adipocytes [12], [13].
Glucose toxicity: when gaining body fat is better than storing glucose
Continuous exposure to higher blood glucose levels can eventually lead to glucotoxicity – an impaired ability of the beta cells of the pancreas to produce enough insulin to store away glucose. This leads to an elevated fasting glucose level and consequently elevated fasting triglycerides (as mentioned before, increases blood glucose levels stop fat metabolism). When the blood is stuffed with fat, hormonal signaling is diminished and resistance to the hormonal signals occurs. In the case of diets, the most common is insulin resistance [14]. This vicious cycle leads to further fat accumulation, increased blood lipid and glucose levels, increased inability to metabolize body fat, increased hunger, increased blood pressure, inflammation, etc. Interestingly, while glucotoxicity seems to a negative state, it is actually a protection mechanism to prevent structural damage to the cells and the DNA, from the reactive oxygen species produced by glucose metabolism.
Fructose and Fatty Liver
Fructose is in a natural environment provided through fruits and vegetables, where it is usually stored in combination with glucose in the form of sucrose. However, the primary source of fructose in today’s diets comes from sweeteners such as table sugar and high-fructose corn syrup. These provide an easy way to consume large quantities of fructose, which has been linked to many modern metabolic diseases [15].
Storing glucose is easy
Compared to glucose, fructose is transported to the cells passively i.e. without the powerful assistance of a hormone, as glucose has with insulin. Therefore, the rate of its uptake is much slower and less tolerable [15]. When we compare how much glucose and fructose we can store in our blood, we see that storage capacity for glucose is almost 1000 times higher (normal fasting levels start at 4 mmol\L) compared to fructose (normal fasting levels start at 0.005mmol\L) [16, p. 17].
Liver overdose: When the body just can't handle the amount
To make up for the lack of efficiency of active transport, fructose is metabolized predominantly in the liver. However, to prevent liver overload, the small intestines absorb and convert a part of ingested fructose into glucose (~42%), lactate (~20%), alanine (~10%), and other organic acids (~3%) beforehand [17], [18]. Based on the calculations using a rat model, around 14% of fructose passes through the intestinal tract intact for further metabolism in the liver [18]. Similarly, the remainder of the fructose being metabolized in the liver, it is mostly converted to glucose and lactate, and in smaller percentages also into triglycerides. However, because of the rate-limiting ability of the intestines to absorb/clear fructose (small dosages can be cleared by ~90% while larger ones only by ~30%), higher dosages lead to more fructose being metabolized in the liver. It is on that occasion, where fat deposits can accumulate in the liver itself.
Liver failure: it all starts with a fatty liver
A condition, where the triglycerides in the liver are not efficiently burned for energy and so remain in the liver is called non-alcoholic fatty liver disease [19]–[22]. In this condition, the liver gets enlarged (by 5 – 6%), because of fat deposits. This leads to inflammation, cell death, and scarring i.e. steatohepatitis. If the inflammation is not neutralized blood flow is reduced and scar tissues are formed in the liver – fibrosis. Eventually, fibrosis can lead to a more severe stage called cirrhosis. In this stage, many severe side effects can be observed, such as fluid retention in the abdomen – ascites, swelling of veins which can rupture and bleed, liver cancer, and eventually liver failure.
Continuous exposure to higher blood glucose levels can eventually lead to glucotoxicity - an impaired ability of the beta cells of the pancreas to produce enough insulin to store away glucose.
Conclusion
In this post we have looked at how the two most prevalent carbohydrates: glucose and fructose, are stored as excess fat in our adipocytes (body fat) or our liver. Both of the sugars are acquired from plants, either as a result of breaking down starches ( tubers and grains) or directly from the food (fruits, vegetables and especially sweeteners).
We have seen that our blood has a limited storage capacity for both sugars, so there are mechanisms to prevent high levels. Diving deeper into the metabolic pathways we have described how glucose is predominantly stored as glycogen and eventually as body fat (when glycogen reserves are full), while fructose itself is in part converted and stored as triglycerides in the liver, which can lead to more severe health complications.
References
[1] H. Elrick, L. Stimmler, C. J. Hlad, and Y. Arai, “Plasma Insulin Response to Oral and Intravenous Glucose Administration,” J. Clin. Endocrinol. Metab., vol. 24, no. 10, pp. 1076–1082, Oct. 1964, doi: 10.1210/jcem-24-10-1076.
[2] T. Yu, B. S. Jhun, and Y. Yoon, “High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission,” Antioxid. Redox Signal., vol. 14, no. 3, pp. 425–437, Feb. 2011, doi: 10.1089/ars.2010.3284.
[3] M. J. Franz, “The Glycemic Index: Not the most effective nutrition therapy intervention,” Diabetes Care, vol. 26, no. 8, pp. 2466–2468, Aug. 2003, doi: 10.2337/diacare.26.8.2466.
[4] D. A. Baur et al., “Adipose Lipolysis Unchanged by Preexercise Carbohydrate Regardless of Glycemic Index,” Med. Sci. Sports Exerc., vol. 50, no. 4, pp. 827–836, Apr. 2018, doi: 10.1249/mss.0000000000001498.
[5] M. V. L. D. Silva and R. C. G. Alfenas, “Effect of the glycemic index on lipid oxidation and body composition,” Nutr. Hosp. Organo Of. Soc. Esp. Nutr. Parenter. Enter., vol. 26, no. 1, pp. 48–55, 2011.
[6] H. Chen et al., “Advanced glycation end products increase carbohydrate responsive element binding protein expression and promote cancer cell proliferation,” Mol. Cell. Endocrinol., vol. 395, no. 1, pp. 69–78, Sep. 2014, doi: 10.1016/j.mce.2014.07.021.
[7] B. R. Landau, J. Wahren, V. Chandramouli, W. C. Schumann, K. Ekberg, and S. C. Kalhan, “Contributions of gluconeogenesis to glucose production in the fasted state.,” J. Clin. Invest., vol. 98, no. 2, pp. 378–385, Jul. 1996, doi: 10.1172/JCI118803.
[8] A. C. Beynen, W. J. Vaartjes, and M. J. H. Geelen, “Opposite Effects of Insulin and Glucagon in Acute Hormonal Control of Hepatic Lipogenesis,” Diabetes, vol. 28, no. 9, pp. 828–835, Sep. 1979, doi: 10.2337/diab.28.9.828.
[9] J. W. Farquhar, A. Frank, R. C. Gross, and G. M. Reaven, “Glucose, insulin, and triglyceride responses to high and low carbohydrate diets in man.,” J. Clin. Invest., vol. 45, no. 10, pp. 1648–1656, Oct. 1966.
[10] T. Scherer et al., “Brain Insulin Controls Adipose Tissue Lipolysis and Lipogenesis,” Cell Metab., vol. 13, no. 2, pp. 183–194, Feb. 2011, doi: 10.1016/j.cmet.2011.01.008.
[11] P. Sonksen and J. Sonksen, “Insulin: understanding its action in health and disease,” Br. J. Anaesth., vol. 85, no. 1, pp. 69–79, Jul. 2000, doi: 10.1093/bja/85.1.69.
[12] R. M. McDevitt, S. J. Bott, M. Harding, W. A. Coward, L. J. Bluck, and A. M. Prentice, “De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women,” Am. J. Clin. Nutr., vol. 74, no. 6, pp. 737–746, Dec. 2001, doi: 10.1093/ajcn/74.6.737.
[13] J. R. Krycer et al., “Insulin signalling requires glucose to promote lipid anabolism in adipocytes,” J. Biol. Chem., p. jbc.RA120.014907, Jul. 2020, doi: 10.1074/jbc.RA120.014907.
[14] S. Kawahito, H. Kitahata, and S. Oshita, “Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress,” World J. Gastroenterol. WJG, vol. 15, no. 33, pp. 4137–4142, Sep. 2009, doi: 10.3748/wjg.15.4137.
[15] S. A. Hannou, D. E. Haslam, N. M. McKeown, and M. A. Herman, “Fructose metabolism and metabolic disease,” J. Clin. Invest., vol. 128, no. 2, pp. 545–555, Feb. 2018, doi: 10.1172/JCI96702.
[16] M. T. Le et al., “Effects of high fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects,” Metabolism, vol. 61, no. 5, p. 641, May 2012, doi: 10.1016/j.metabol.2011.09.013.
[17] C. Jang et al., “The small intestine shields the liver from fructose-induced steatosis,” Nat. Metab., vol. 2, no. 7, Art. no. 7, Jul. 2020, doi: 10.1038/s42255-020-0222-9.
[18] C. Jang et al., “The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids,” Cell Metab., vol. 27, no. 2, pp. 351-361.e3, Feb. 2018, doi: 10.1016/j.cmet.2017.12.016.
[19] L. Tappy and K.-A. Lê, “Metabolic Effects of Fructose and the Worldwide Increase in Obesity,” Physiol. Rev., vol. 90, no. 1, pp. 23–46, Jan. 2010, doi: 10.1152/physrev.00019.2009.
[20] M. J. Dekker, Q. Su, C. Baker, A. C. Rutledge, and K. Adeli, “Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome,” Am. J. Physiol.-Endocrinol. Metab., vol. 299, no. 5, pp. E685–E694, Sep. 2010, doi: 10.1152/ajpendo.00283.2010.
[21] M. B. Vos and J. E. Lavine, “Dietary fructose in nonalcoholic fatty liver disease,” Hepatology, vol. 57, no. 6, pp. 2525–2531, 2013, doi: 10.1002/hep.26299.
[22] M. F. Abdelmalek et al., “Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease,” Hepatology, vol. 51, no. 6, pp. 1961–1971, 2010, doi: 10.1002/hep.23535.

Simon Knez
Java Software Developer, High Intensity Training Exercise Specialist, Carnivore Diet Coach